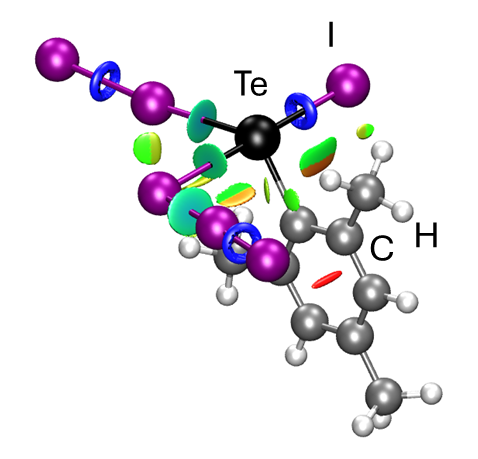
Chalcogen-containing molecules have peculiar properties. They are generally considered "soft" donor atoms under the HSAB principle. This can mostly be attributed to a larger polarizability and a more diffuse nature of the p-orbitals. Simultaneously, the degree of s/p-orbital mixing decreases when moving from sulfur to selenium and from selenium to tellurium. A consequence is a more pronounced directionality despite the potential size mismatch with smaller ("harder") metal ions or main-group bonding partners. A prominent feature of the Σ-bonds formed by the heavier chalcogens is the presence of a σ-hole — a localized electropositive region on the backside of the atom engaging in a σ-bond caused by the corresponding anti-bonding σ*-orbital — that allows for non-covalent interactions.
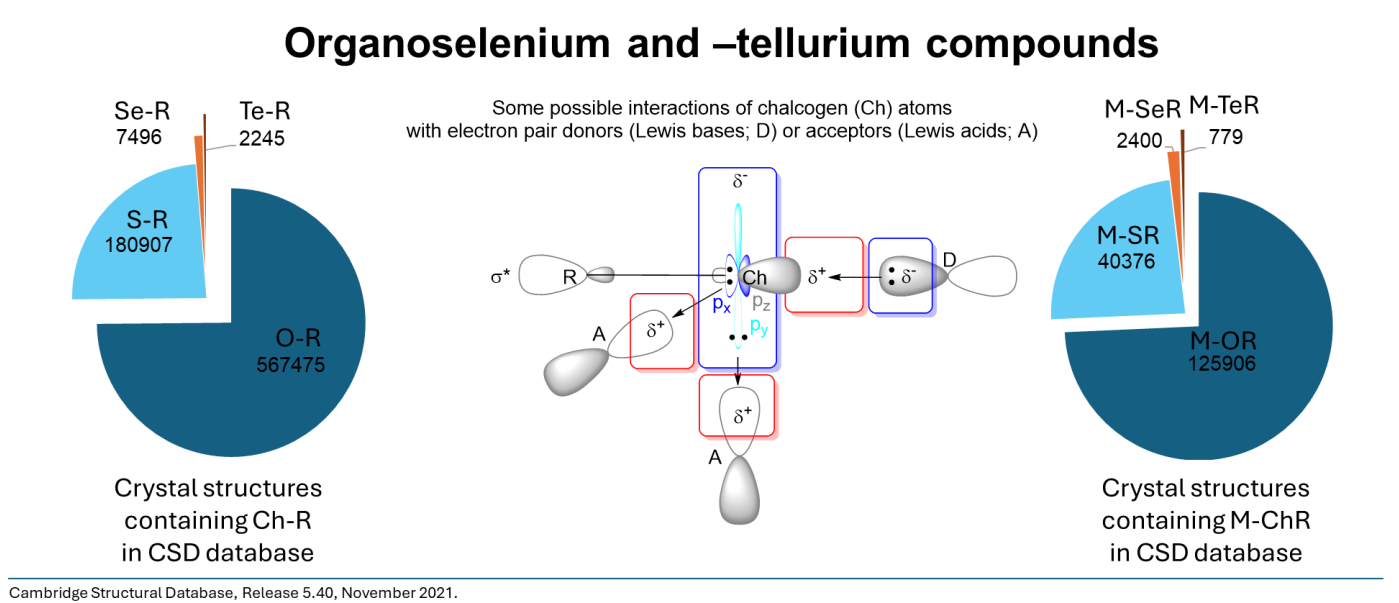
Parallelly, chalcogen donor ligands can principally be tailored electronically and may represent a potentially more robust alternative to more established but rather sensitive, tailorable chelators, such as phosphine or carbene donor ligands. We are interested in all aspects of chalcogen chemistry (e.g., non-covalent interactions), as well as in chelator design and the chemistry of simple monodentate ligands. Chalcogen-containing chelators are studied in close cooperation with researchers from Brazil, where we also contribute significant computational expertise.