A research laboratory led by Maximilian Roca Jungfer dedicated to
Our Motivation
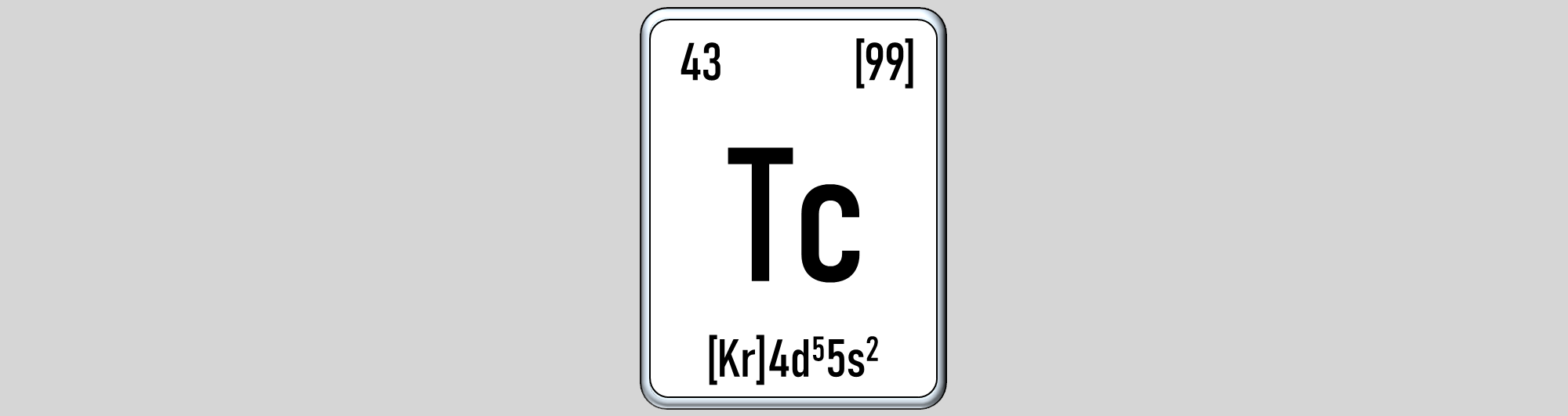
Technetium exists scarcely on Earth. Traces of the element had been formed by spontaneous fission of U-235, while it has also been identified to naturally occur in stars. Adverse releases, such as those related to nuclear weapons or accidents have caused an increase in the abundance of environmental technetium. The most commonly used technetium nuclide, Tc-99, accumulates with a high overall fission yield in spent nuclear fuel. Due to its long half-life, it is therefore relevant for nuclear waste. Simultaneously, many radiopharmaceuticals rely on its nuclear isomer Tc-99m, which is a diagnostic workhorse in nuclear medical imaging. Understanding the fundamental chemistry of Tc, thus, forms not only the necessary basis for developments in geo- and eco-chemistry but also for applications in nuclear medicine. Scoping differences between technetium and its homolog rhenium, which is often used as a non-radioactive surrogate, will lead to new insights with potential implications and relevance to more applied fields in the long term.

Our Approach
With a diverse background in organometallic
and coordination chemistry, we seek to
expand the fragmented field of
(organo)technetium chemistry. Applying and
coupling modern techniques, such as
multinuclear nuclear magnetic resonance
(NMR) spectroscopy, attenuated total
reflection Fourier-transform infrared
(ATR-FT-IR) spectroscopy, and advanced X-ray
absorption spectroscopy (XAS; XANES/EXAFS)
with structural information obtained from
diffraction experiments, we are working at
the interface of preparative molecular
organic and inorganic chemistry,
crystallography and spectroscopy —
supplemented and guided by quantum chemical
calculations.
A special focus on the
development of model systems allows the
systematic study of chemical phenomena,
providing comprehensive insights into
bonding properties, electronic structure and
reactivity.