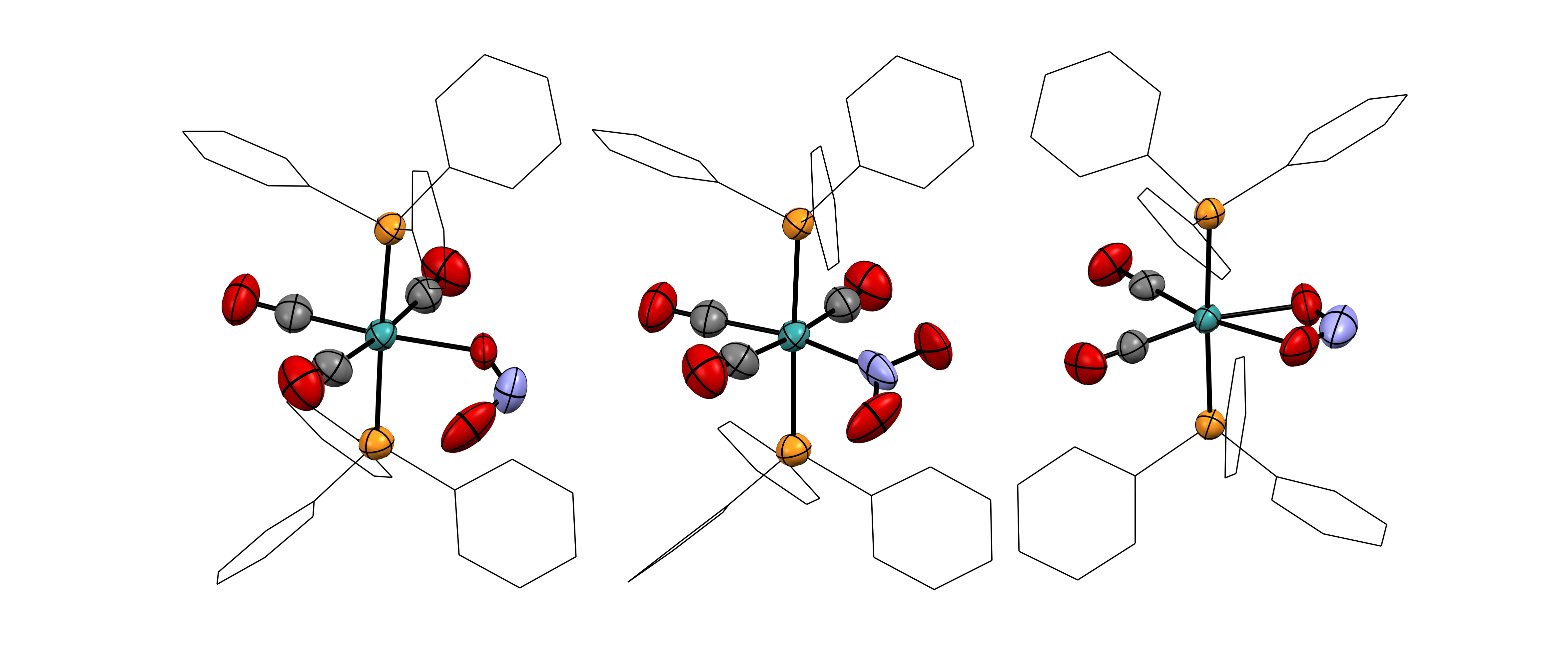Element number 43
Technetium
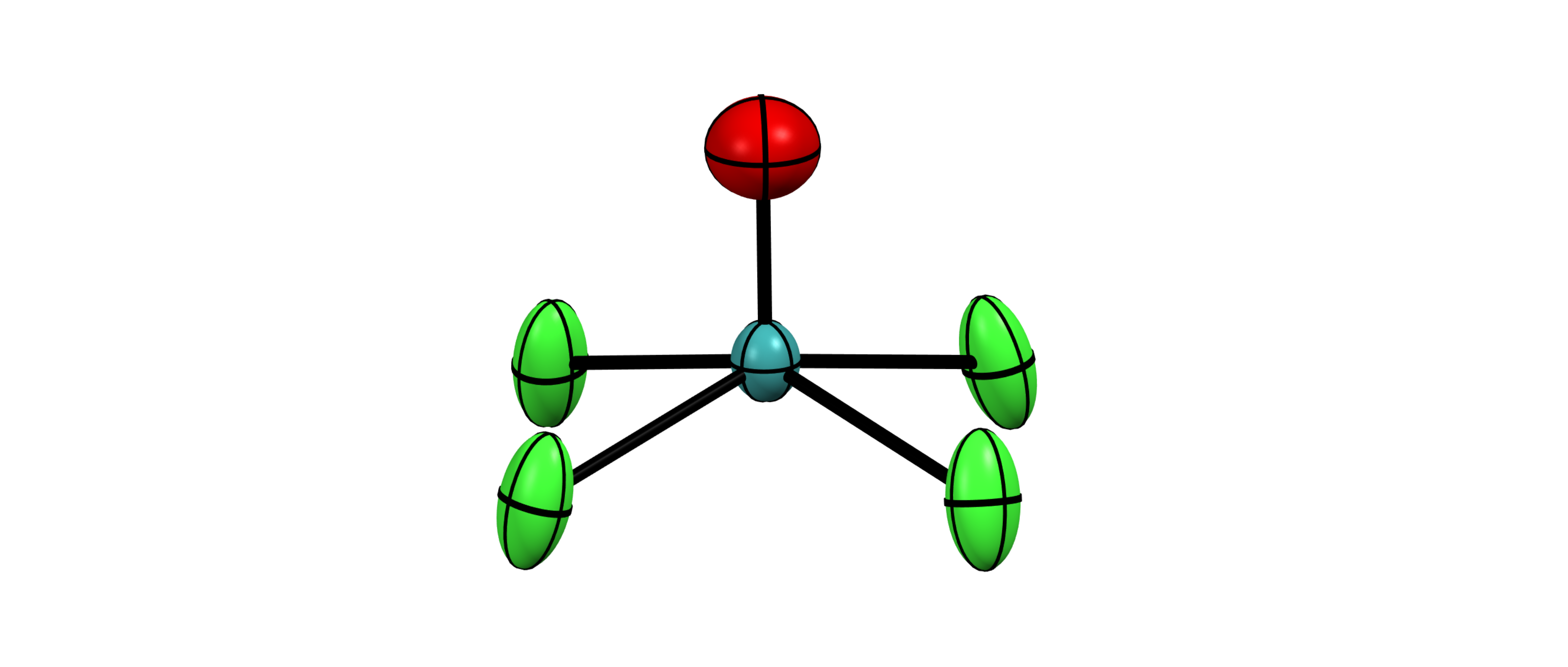
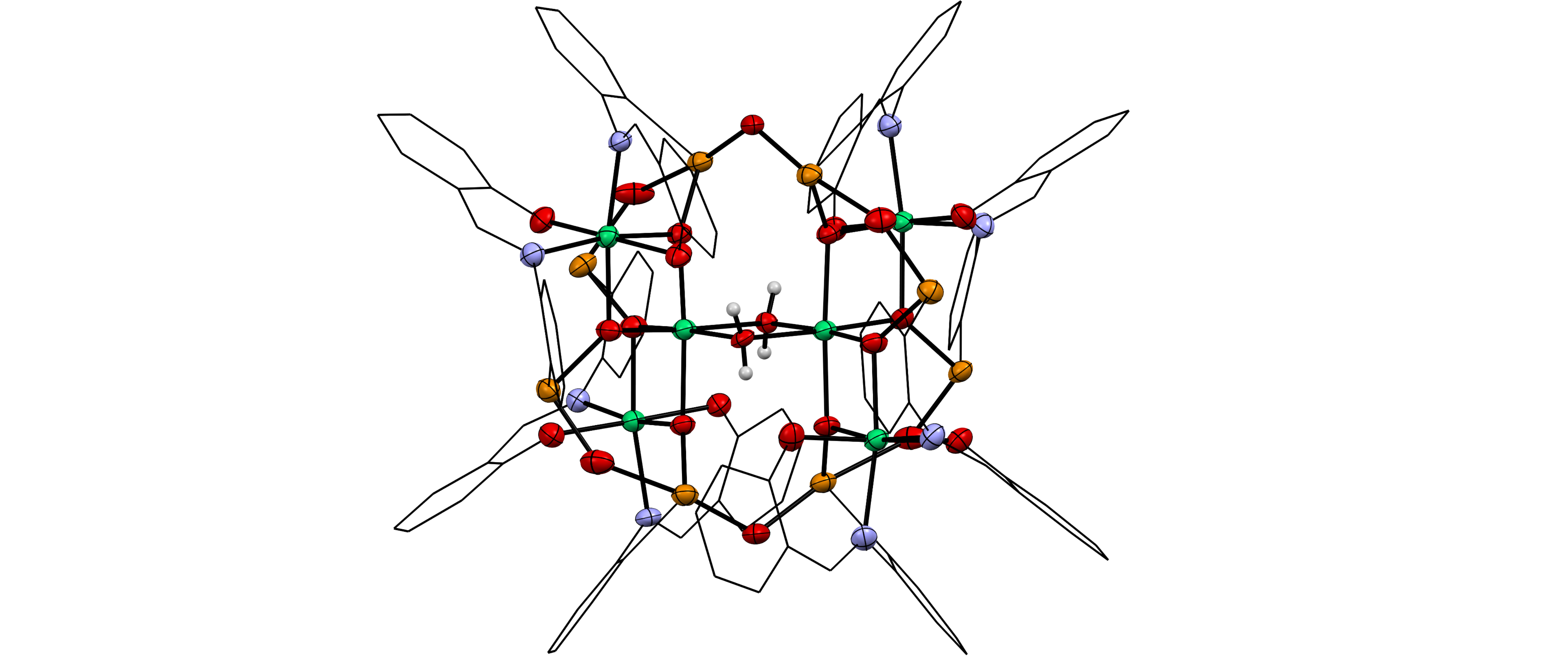
Nearly non-existent in nature, the radioelement technetium was first discovered in neutron-activated molybdenum as late as 1937. Significant advancements in technetium chemistry have often been driven by its undisputed importance in nuclear medicine and its relevance for nuclear waste. Such achievements were, however, generally preceded by a foundation of breakthrough discoveries in the fundamental chemistry of the element that resulted from less application-focused research. In this regard, even the general chemistry with simple ligands such as halido or oxido ligands is relatively poorly understood to this date, while major discoveries continue being reported in recent years. Motivated by the special position of technetium in the middle of the periodic table, we study and develop the fundamental chemistry of this fascinating element using modern tools, such as X-ray crystallography, multinuclear nuclear magnetic resonance (NMR) spectroscopy and advanced X-ray spectroscopy combined with predictions based on quantum chemical calculations.